Compounds that have the exact same number of atoms, i.e., the identical empirical formula, but differ from one another by the arrangement of the atoms is known as Isomers.
Types of isomers
Isomerism can be divided into two main subcategories, each of which has a different definition. Stereoisomerism and structural isomerism are the two main forms. The taxonomy of several isomer kinds is shown below.
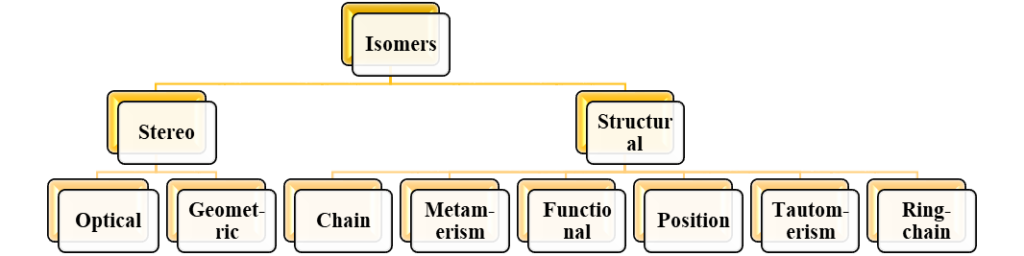
Structural
Constitutional isomerism is the name given to structural isomerism. In the molecules of these isomers, the atoms and functional groups are connected in various ways. Since distinct structural isomers might or might not include the same functional group, they are given unique IUPAC designations.
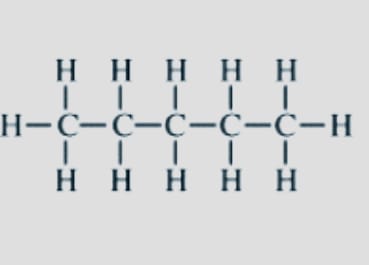
- Chain Isomers
Also known as Skeletal isomerism. These isomers constituents have variously branching architectures. Frequently, the branching of the carbon in chain isomers varies.
- Metamerism
Metamers are isomers with the same chemical formula on both sides of functional groups but distinct alkyl groups. The existence of several alkyl chains on either side of the functional group causes this kind of isomerism to occur.
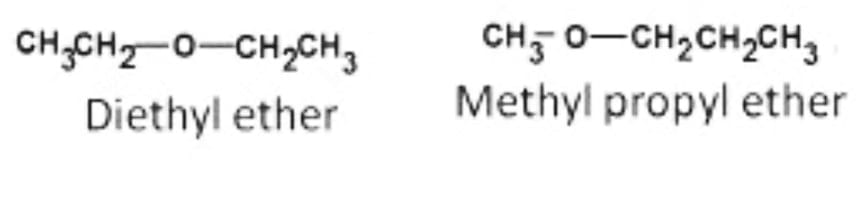
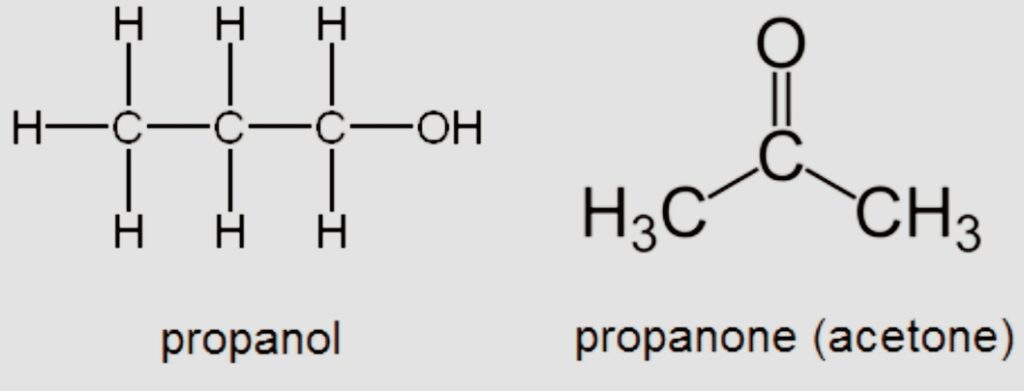
- Functional
Also known as functional group isomerism. As implied by the name, it describes substances with the same chemical formula but various functional groups linked to them.
- Position
Constitutional isomer that differ from one another by the position of the functional groups on or in the carbon chain despite sharing the identical carbon skeleton and functional groups.
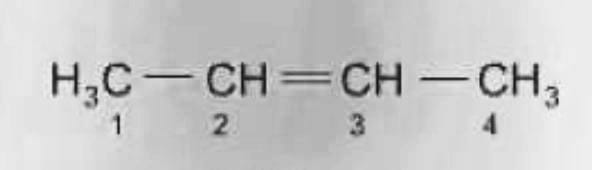
- Tautomerism
The compound isomer that merely vary in the protons and electrons positions. The tautomer of a molecule typically coexist in equilibrium and are readily interchangeable.
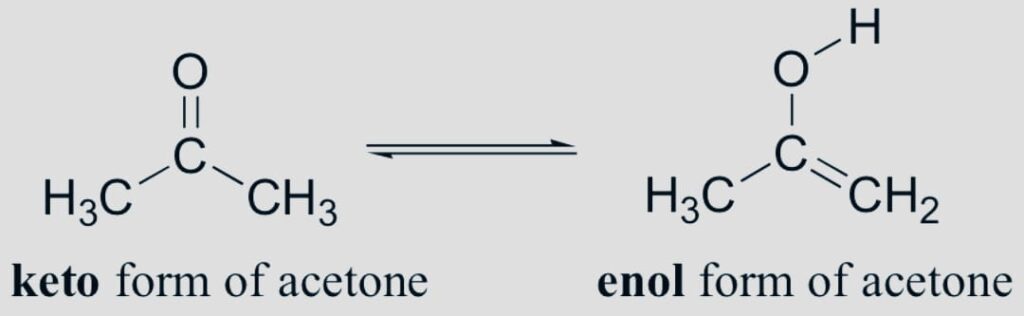
- Ring-chain
In chemistry ring-chain isomers are compounds with the same chemical formula but different open chain and cyclic structures. They typically differ in the quantity of pi bonds present.
Stereoisomerism
Compounds with the same chemical formula but varying orientations of the molecule’s atoms in three-dimensional space exhibit this sort of isomerism. Two subtypes of this phenomenon are further classified. In this subsection, both of these categories are briefly explained.
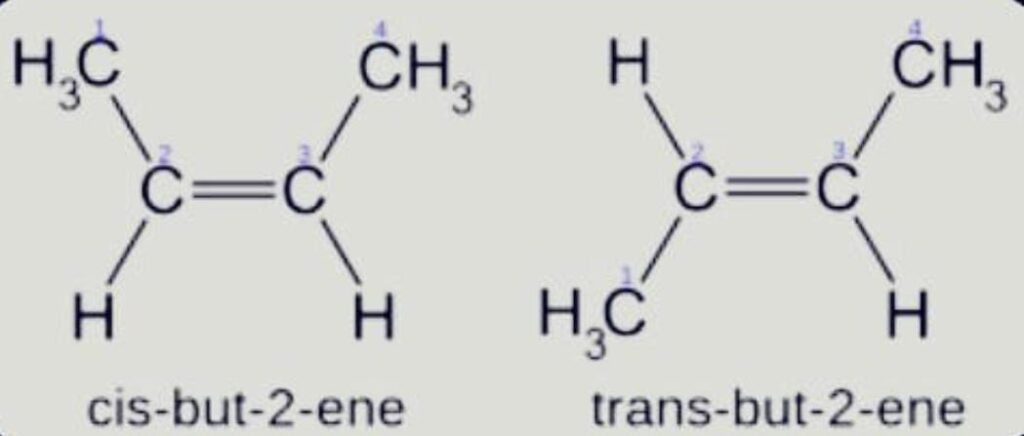
- Geometrical
Isomer with the same atom bonding order but a distinct spatial arrangement of the atoms. It’s commonly referred as cis-trans isomerism.
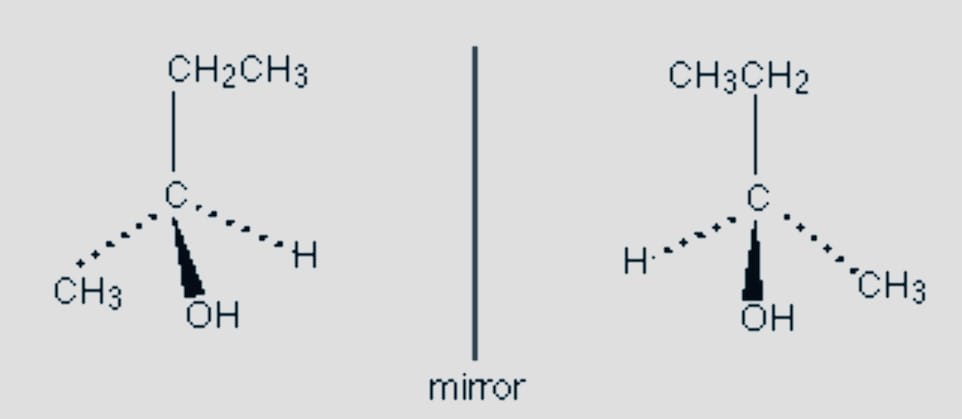
- Optical
Another kind of stereoisomerism is optical, sometimes referred to as enantiomers. This happens when molecules with the same structural formula and molecular formula cannot combine to form a new molecule.
Also Read About:

