Isotopes are defined as elements with the same atomic number but a different mass number.
The three hydrogen isotopes that can be found in nature are;
- Tritium
- Deuterium
- Protium
- The periodic table’s initial element, hydrogen, has an atomic number one. Protium 11H, deuterium 21H or D, and tritium 31H or T are the three hydrogen isotopes. Because the isotopes have varying amounts of neutrons, they are different.
- There are no neutrons present in protium, whereas there is one neutron in deuterium and two in tritium.
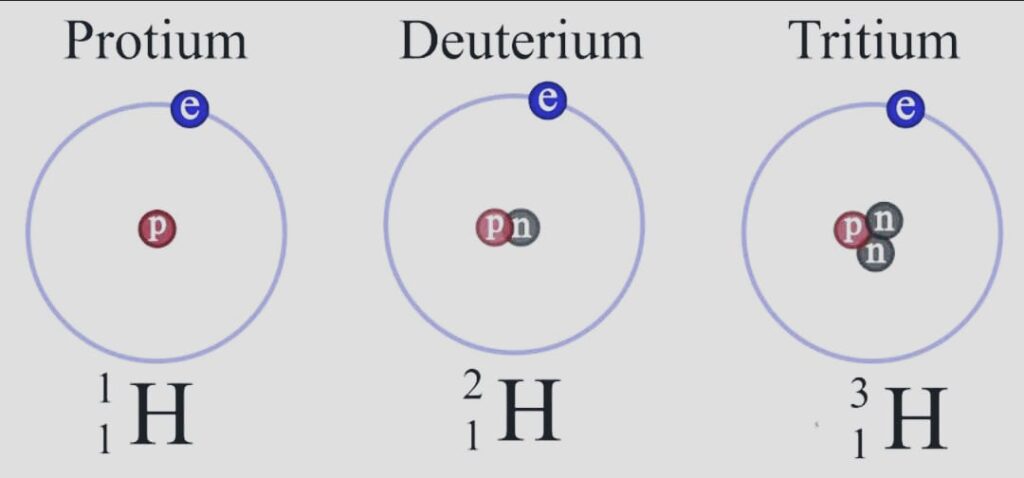
- Deuterium, which makes up 0.0156% of the hydrogen on the earth’s surface, is the most prevalent form of the element.
- One tritium atom is present for every 1018 protium atoms.
- Only tritium, which emits low-energy beta particles, is radioactive among these three hydrogen isotopes. Since all isotopes have the same electrical arrangement, they all have comparable chemical properties.
- However, due of the various bond disassociation enthalpies, they have varying rates of reactivity. The wide variances in mass give them different physical characteristics.
Protium (11H)
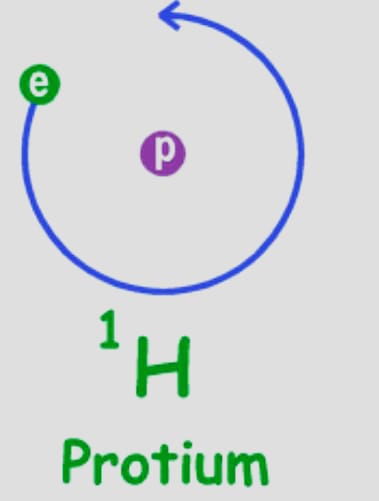
It is one of the more prevalent hydrogen isotopes. Nature is abundant, with a 99.98% surplus. One of the causes of this is that this isotope’s nucleus only has one proton, and it has never been recorded that this proton has decayed.
Protium has a mass of 1.007825 amu. In compounds, hydrogen typically joins up with other atoms to form H2 ( diatomic hydrogen gas).
Deuterium (21H)
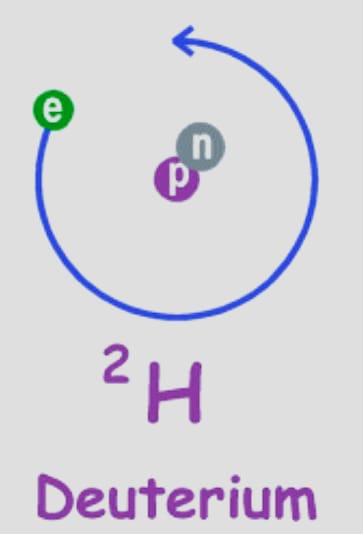
Its nucleus contains a proton and a neutron. The deuteron is the name for the hydrogen-2 nucleus. It doesn’t emit radiation. Its compounds are utilized as solvents for hydrogen 1 and in chemical analysis. Instead of protium-based molecules, heavy water is enhanced with deuterium-based ones.
It functions as a neutron moderator and coolant. Nuclear fusion also uses hydrogen 2 as a fuel (commercial). As the gas deuterium, it naturally occurs.
Tritium (31H )
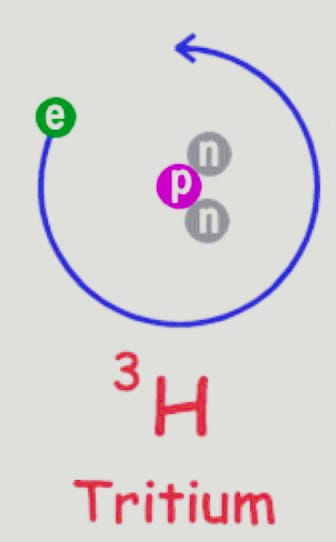
Its nucleus contains one proton and two neutrons. The interaction of cosmic rays with atmospheric gases results in minute amounts of tritium, often known as hydrogen 3, appearing in nature. Additionally, they are briefly released when nuclear weapons are tested.
It is radioactive and undergoes beta decay, which produces helium 3. The atomic mass of hydrogen 3 is 3.0160492 u.
Also Read About;




