The stronger bonds in chemistry are known as covalent bonds. These bonds are formed when an electron is shared between two elements. Covalent bonds are the strongest and most common form of chemical bonding.
The covalent bond is stronger than any other bond because the electron pairs are close together. Covalent bonds are formed when atoms share their valence electrons. This completes the outer shell of the atom and nuclei are formed around the atom. Since each of the electrons is paired here, it is very difficult to break the bond.
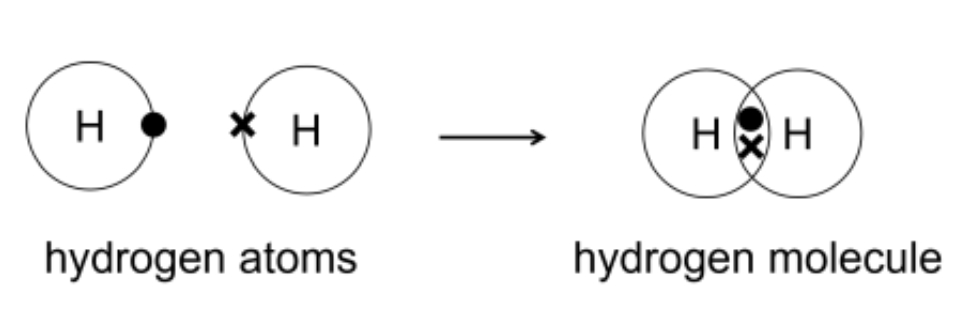
The strength of a bond between two atoms increases with the number of electron pairs in the bond.

