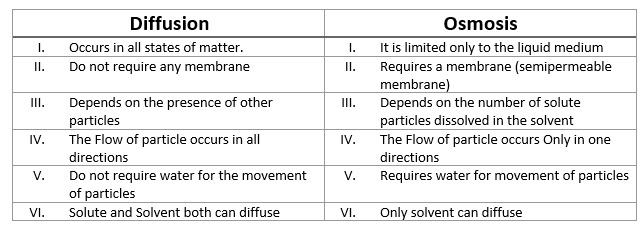
There is a remarkable difference between Osmosis and Diffusion. For instance:
- Diffusion occurs in all states of matter, Solid, Liquid and Gas. While, Osmosis is only restricted for Liquid.
- Osmosis requires a membrane known as Semipermeable membrane. Whereas, Diffusion does not require any membrane.
- In Osmosis process only the solvent molecules can diffuse. But in Diffusion both solvents and solutes molecules can be diffused.
Comparison can also be seen as followed: