Understanding the Titration
In chemistry, Titration is a method for determining the amount of a drug A by adding calibrated amounts of a substance B, the titrant with which it interacts, until an exact chemical equivalent (the equivalence point) is reached.
Titration Formula
- The type of reaction determines the indication used. Chemists commonly use phenolphthalein or methyl orange as indicators in acid-base titrations.
- Titrations are most commonly used to determine the unknown amount of a component (the analyte) in a solution by allowing it to react with the solution of another compound (the titrant).
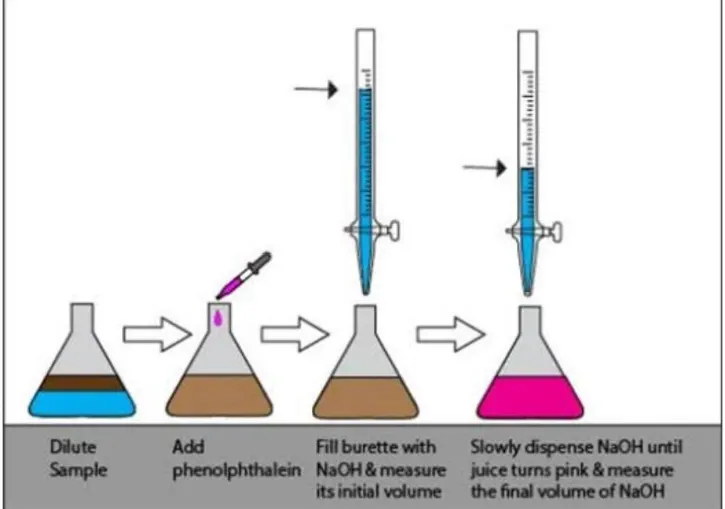
Types Of Titration
It comprises four distinct categories.
- Acid-base titrations.
- Redox titrations.
- Titrations of precipitates.
- Complexometric titrations.
ACID-BASE TITRATIONS
Analysts use a standard base solution to evaluate an acid’s potency through acidimetry. It contains an acid-base neutralizing process, with water acting as the solvent. Hydrogen and the hydroxide ion combine in this process to create water.
H+ + OH– → H2O
REDOX TITRATIONS
Scientists (or Chemists) often abbreviate oxidation-reduction reactions as redox reactions. Titration uses reactions that happen as a result of electron transfers between reacting solutions that are either oxidizing or reducing agents.
- The oxidizing agent is KMnO4
- The reducing agent is Na2S2O3
COMPLEXOMETRIC TITRATIONS
A weakly dissociated complex substance is created by titrating a metal-ion solution with a complexing agent. Analysts often use ethylene-diamine tetraacetic acid (EDTA) titration to determine metal ion concentrations.
Ag + 2 CN → [Ag(CN)2]
PRECIPITATION TITRATIONS
Precipitation titration is based on the insoluble precipitate that forms when the two reacting chemicals come into contact.
Further Read About:

