Understanding Molecular Structures and Chemical Bonds
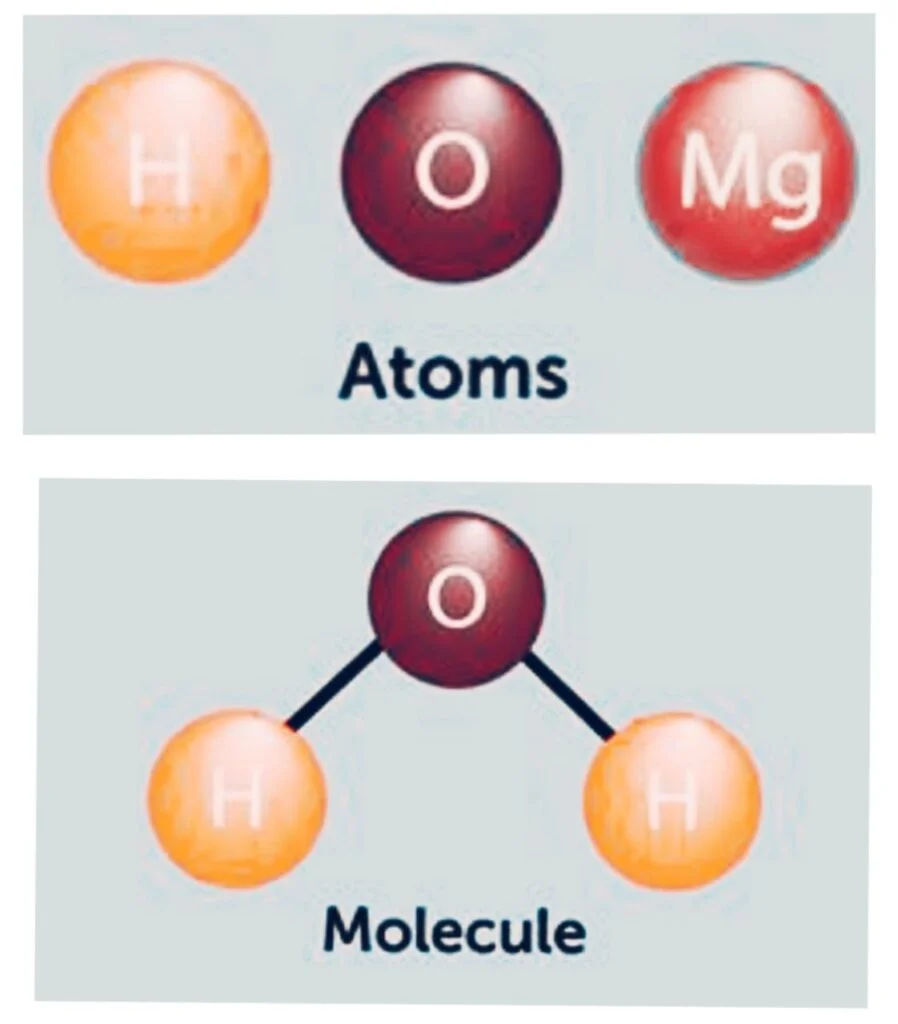
The smallest component of a compound, a molecule, holds the chemical characteristics of the compound.
The lowest fundamental unit of a chemical compound that can participate in a chemical reaction is a group of bound atoms.
- Groups of atoms make up molecules.
- An atom’s structure is also broken into smaller components. The constituent parts of an atom are protons, electrons, and neutrons. The atom’s nucleus houses the protons and neutrons, and electrons surround the nucleus.
- In contrast to electrons, which have a negative charge, protons have a positive charge.
- There is no charge in a neutron.
- The existence of protons explains why the nucleus is a positively charged ion.
- An atom’s nucleus is the central mass of the atom. Most atoms are empty.
- There is a specific atomic number for each element.
- The quantity of protons in an element’s nucleus is known as the element’s atomic number. Z stands for it.
Atomic mass considers the mass of an atom’s constituent particles. The mass of an electron is quite little. Thus, the mass of an atom is equal to the product of its protons and neutrons masses. Indicating the mass number is A.
Molecule Examples
A molecule is the smallest recognizable unit of a pure material that retains its composition and chemical properties, consisting of two or more atoms. Several types of molecules include:
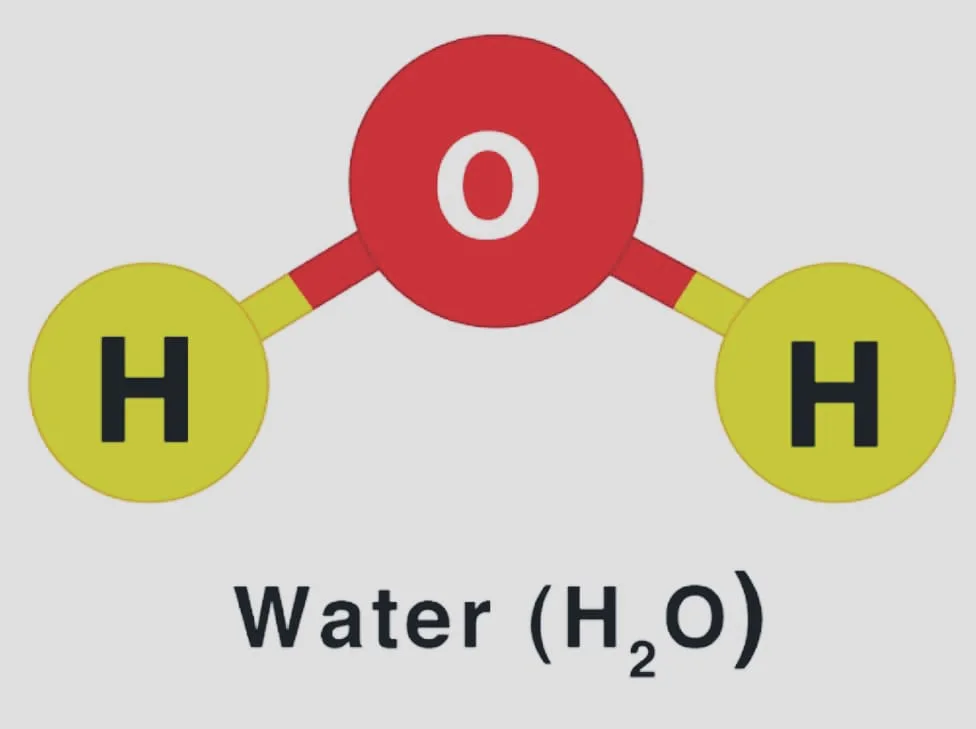
- H2O (Water)
- HCl (Hydrogen Chloride)
- NaCl (table salt)
- CO2 (Carbon dioxide)
- C₆H₁₂O₆(glucose, a type of sugar)
- O₃ (ozone)
- CaO (calcium oxide)
- One hydrogen atom joins with one chlorine atom to form the HCl(g) molecule, a diatomic molecule with only two atoms.
- An NaCl(s) crystal forms an ionic lattice with an indefinite number of atoms instead of a distinct molecule.
- Two oxygen atoms bond with a carbon atom to form carbon dioxide (CO₂), a triatomic molecule with three atoms.
Further Read About:

