Understanding Atomic Structure and Isotopes
Isotope is the variations of chemical elements that have a varied number of neutrons but the same number of protons and electrons. As a result of a difference in the total number of neutrons in their respective nuclei.
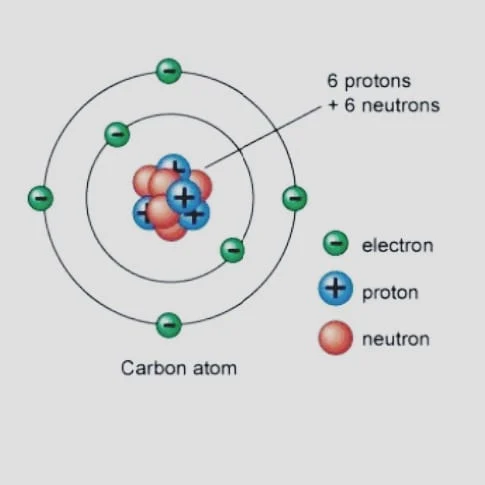
Properties
- When discussing an element’s isotopes chemical properties, they are almost equal or the same. Isotopes behave chemically in essentially the same ways.
- However, there are differences among isotopes when it comes to their physical characteristics, such as their mass, melting or boiling temperature, density, and freezing point.
- The mass of each isotope has a significant impact on its physical characteristics. We can identify different isotopes thanks to their differences.
Types of Isotope
- Isotopes exist for every element.
- Stable and unstable isotopes are the two basic categories (radioactive).
- 254 stable isotopes are known.
- Scientists refer to all synthetic (lab-made) isotopes as radioisotopes since they are radioactive due to their instability.
- Certain substances can only exist in unstable states (for example, uranium).
Examples of Isotope
Some examples are discussed below;
Carbon atoms have two isotopes respectively Both have six protons, but there is a difference of 6 in 12C and 8 in 14 C. While carbon-14 is a radioactive isotope, carbon-12 is a stable isotope (radioisotope).
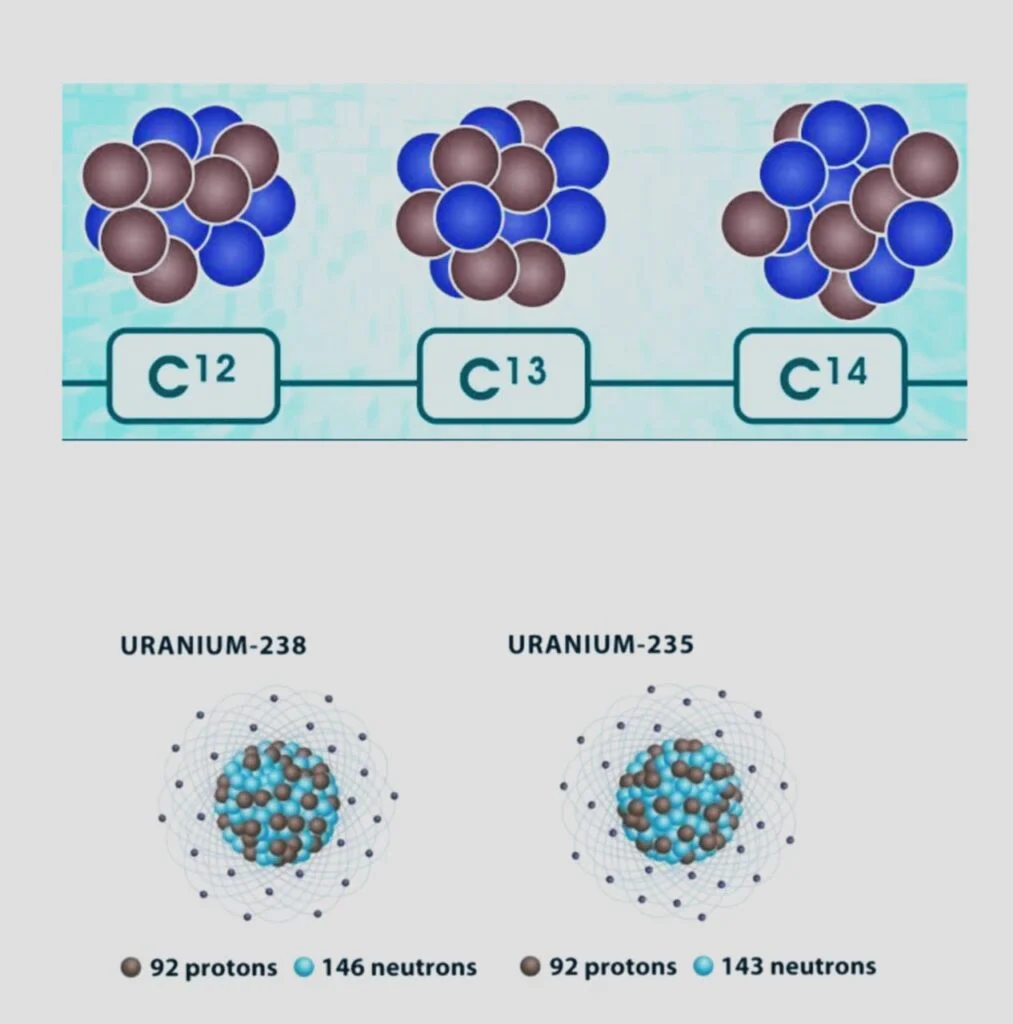
In the crust of the Earth, uranium-235 and uranium-238 are found naturally. Each has a prolonged half-life. As a byproduct of decay, uranium-234 is created.
Carbon and hydrogen are two typical isotopes. There are three stable isotopes of hydrogen atom: protium, deuterium, and tritium. Protium has zero neutrons, deuterium or heavy water has one, and tritium has two neutrons in their nucleus, while all of these isotopes have the same amount of protons.
These are just a few instances of common isotopes; there are also 22 known isotopes of tin, 21 known isotopes of zinc, 3 known isotopes of neon, 9 stable isotopes in natural xenon, and 14 known isotopes of nickel.
For Further Reading;

