Electronegativity is the propensity of an atom in a molecule to draw the shared pair of electrons toward itself.
- Because it is merely a propensity, this attribute has no dimensions.
- It essentially represents the final consequence of atoms‘ propensities to attract electron pairs that form bonds in various elements.
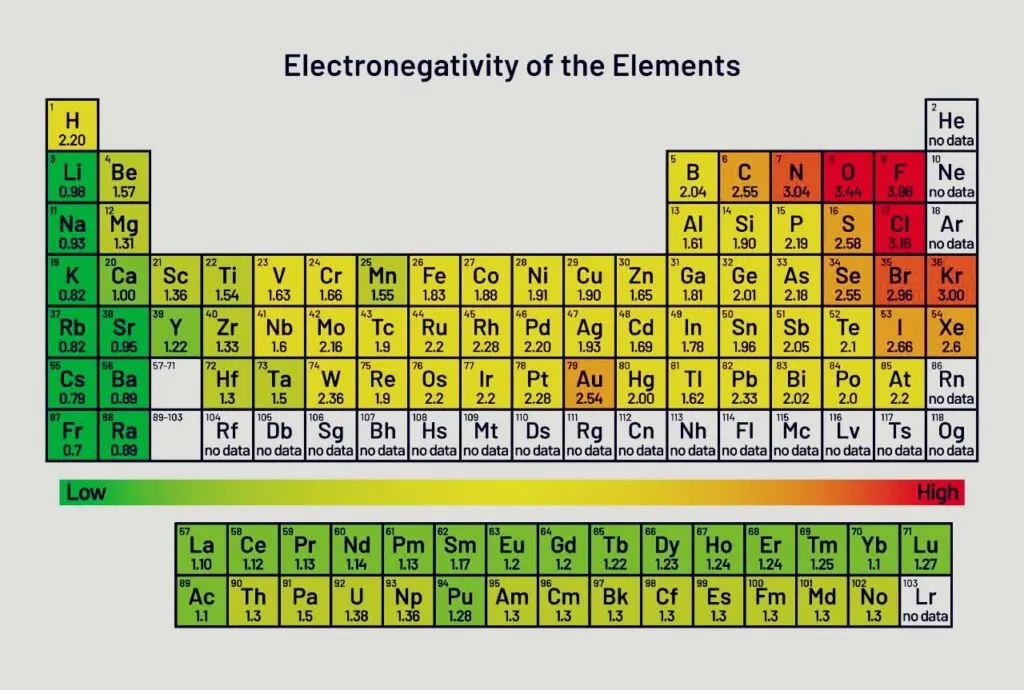
- On several scales, we quantify electronegativity. Linus Pauling created the most widely used scale.
- The least electronegative element is cesium, which has a value of 0.7, and the most electronegative element is fluorine, which has a value of 4.0.
- According to the importance of chemistry, electronegativity will be regarded as the primary factor in chemical bonding and is a significant quantity in defining the nature of bonds between elements.

Electronegativity of Elements: Periodic Trends
- In the modern periodic chart, electronegativity increases across a period as we move from left to right because the nuclear charge increases and the atomic size decreases.
- The periodic table’s electronegativity trend, for instance, is shown below for period 3.
- As we travel down the group in the current periodic table, the atomic number rises.
- Although the nuclear charge likewise rises, the effect of the rise is mitigated by the addition of one shell.
- As we descend the group, the electronegativity value drops.
- For instance, as illustrated in the diagram below, the electronegativity value drops in the halogen group as we proceed from fluorine to astatine.
- A general observation is that metals exhibit lower electronegativity values than non-metals.
- As a result, the nature of metals and non-metals is electropositive and electronegative, respectively.
- Due to their tiny size and greater electronegativity values, the elements in period two have different properties from the elements in their respective groups.
- The second period’s features are similar to those of the following group in period three.
- This is brought on by a little discrepancy in their electronegativities. As a result, a diagonal relationship develops.
Elements that are Most and Least Electronegative
- The periodic table’s most electronegative element is fluorine. It has an electronegativity of 3.98.
- The least electronegative element is cadmium. It has an electronegativity of 0.79.
- Cesium is the most electropositive element because electropositivity is the exact opposite of electronegativity.
- The most electronegative elements are those with the fewest inner electron shells between the positive nucleus and the valence electrons and the fewest required electrons to complete their valence shells.
- Fluorine is the most electronegative of all the elements. It has a 4.0 electronegativity. Electronegativities for metals are less than 2.0.
- With electronegativity values of 0.7, cesium (Cs) and francium (Fr) are the least electronegative elements.
Electronegativity’s Effect on Covalent Bonding
- The electronegativities of the two bound atoms have a significant impact on the covalent bond’s strength (especially the difference in the electronegativities of the bonded atoms).
- Homonuclear diatomic molecules have relatively “pure” covalent bonds because the bound atoms’ electronegativities match (resulting in the bonded pair of electrons being almost equidistant from the two bonded nuclei).
- H2 molecules, Cl2 molecules, and O2 molecules are only a few examples of these covalent linkages in nature.
- On the other hand, polarization often occurs in covalent bonds between two species with different electronegativities.
- This happens as a result of the more electronegative atom drawing the bond pair of electrons closer to itself, which results in the development of a partial negative charge (often represented by the symbol -).
- The more electropositive atom also develops a partial positive charge, represented by the symbol +, at the same moment. The polarity of the chemical connection is caused by these partial charges.
Also Read About:

