Types of Chemical Elements and Their Properties
The substances that cannot be converted into another substance are known as an elements.
- Each type of atom that makes up an element is unique. The chemical elements are all highly distinct from one another because of this.
- Atoms from at least one or more elements are present in everything in the universe.
- The chemistry of elements is organized in order of increasing atomic number, or the quantity of protons in an atom’s nucleus, which typically corresponds with increasing atomic mass, from left to right and top to bottom.
Periodic Table of the Elements
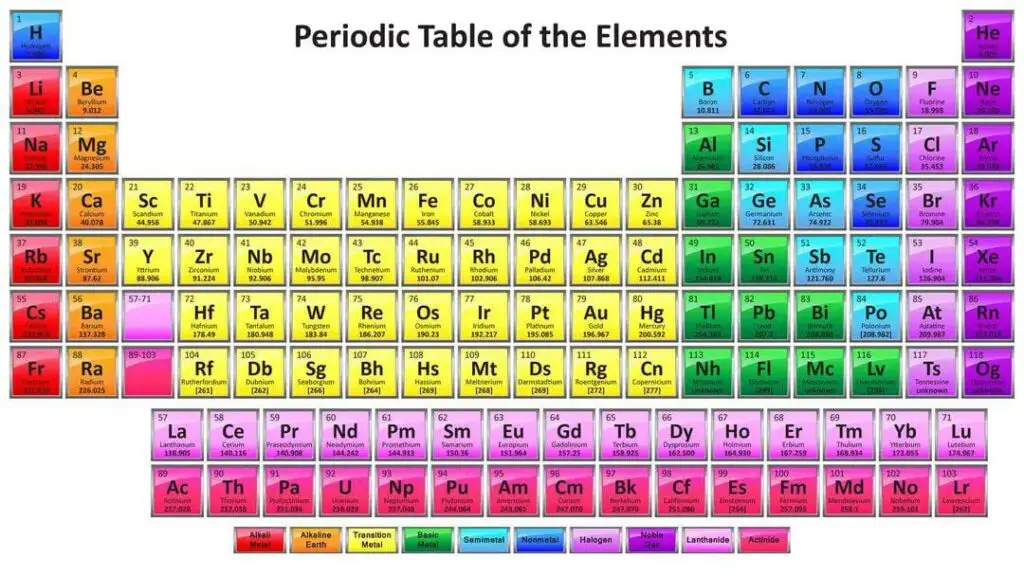
- All known element is listed in the periodic table and those with similar properties are grouped together.
- The majority of them contain isotopes, which are distinct forms of the same element with differing numbers of neutrons but the same number of protons and electrons.
An element consists of identical atoms. For instance, hydrogen consists of hydrogen atoms, oxygen consists of oxygen atoms, and carbon is an element made of carbon atoms. Scientists have discovered 118 elements so far. Elements are categorized into metals and non-metals.
Metallic Elements
They have the following characteristics :
- 1. They are good conductors of heat and electricity.
- 2 . They are shiny
- 3. They are mostly solids, except mercury
- 4. They usually have high melting and boiling points.
- 5. They can be drawn into wires.
- 6. They can be shaped into foil and plates.
Examples: Aluminium , Silver , Gold , Copper , Tin , and Lead.
Non Metal
Non-metals have the following characteristics :
- Non-metals are poor conductors of heat and electricity.
- They are dull.
- They are found in all the three states of matter: solid, liquid, and gas
- They have low melting and boiling points.
- They cannot be drawn into wires or beaten into foil and plates.
Examples: Carbon, Oxygen, Iodine, and Sulphur.
For Further Reading:

