How Electronegativity Influences Chemical Bonding
A chemical feature known as Electronegativity describes an atom’s ability to draw shared-pair electrons to itself within a molecule. Factors affecting electronegativity include atomic size, nuclear charge, and the effect of a substitute.
The electronegativity of atoms on the left and right sides of the periodic table varies significantly. It will be regarded as the primary determinant in chemical bonding because it is a significant quantity in establishing the type of bonds that exist between components.
Below is a list of the elements’ periodic tables, along with a table of their electronegativities.
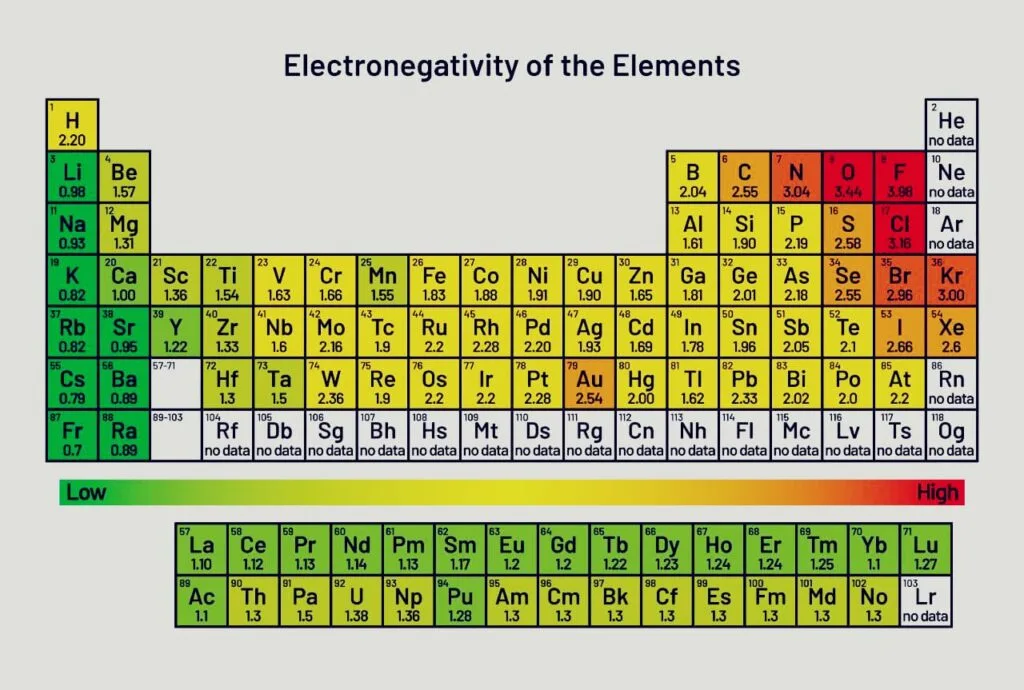
Nuclear charge, shielding, and atomic radius all have an impact on electronegativity. In the periodic table, the electronegativity falls as you move down a group. The electronegativity rises as you move from one era to the next in the periodic table.
Factors affecting electronegativity include the following elements;
Atomic Size
- As an atom’s size increases, its electronegativity falls.
- Since electrons are farther from the nucleus, there is less force of attraction between them, and larger atomic sizes will have lower electronegativity values.
Nuclear Charge
- As nuclear charge rises, electronegativity rises as well.
- A higher electronegativity value will be produced by a higher nuclear charge value.
- This occurs as a result of stronger electron attraction brought on by an increase in nuclear charge.
The impact of the substitute
- An increase in inner electron density tends to decrease electronegativity because of the screening effect.
- The type of substituent that is bonded to an atom determines its electronegativity.
- As opposed to CH₃I, the carbon atom in CH₃I gains a larger positive charge ion.
- As a result, the C-atom in CH₃I is more electronegative than it is in CH₃I. A change in an atom’s electronegativity brought on by substituents alters the atom’s chemical behavior.
For Further Reading:

