Halogens Elements
Nonmetals are halogen. Greek roots give the term “halogens” its original meaning of “salt-forming.”
- Due to their strong reactivity, halogens are only found in the environment in the form of ions or compounds.
- The amount of unpaired valence electrons in the molecule determines the amount of stronger bonds
- Group 17 elements are known as halogens; in their valence shell, they have seven electrons.
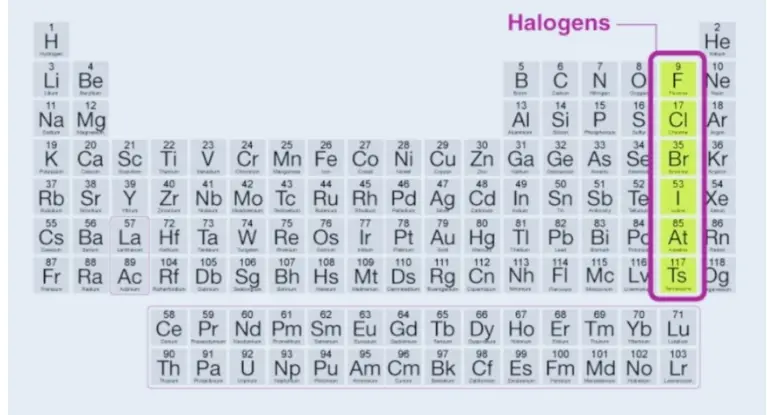
Fluorine, chlorine, and bromine are all gases at ambient temperature, while bromine is a liquid. Astatine and iodine are both solids. Halogens are extremely reactive; from fluorine to astatine, the reactivity diminishes. In nature, halogens do not occur in their elemental form.
Halogens have many properties in common, including the ability to form acids when combined with hydrogen.
Chemical Properties Of Halogen
- All halogen molecules are homonuclear diatomic. This indicates that each of their molecules contains two atoms.
- Halogen form negative ions and are extremely reactive because they contain seven valence electrons instead of six.
- By interacting with the atoms of other elements, they can gain one electron.
- One of the most reactive elements in the universe is fluorine.
- They have intermolecular forces that are generally weak.
Physical Properties
- The only periodic table group with elements in all three of the recognized states of matter at standard pressure and temperature is the group of halogens.
- Fluorine (F) is a colorless gas.
- Chlorine (Cl) is a bluish-green gas.
- The liquid form of bromine (Br) is dark red
- Iodine (I) is a black substance that transforms into a purple vapor when heated.
- A dark solid called astatine (At)
- The halogens all have a powerful and frequently unpleasant odor.
- Halogen substances are quite harmful.
- Ineffective heat and electricity conductors
- Decreased melting and boiling points
- They are all fairly poisonous.
- They combine easily with metals to form salts.
- Their outer shell contains seven valence electrons.
- They have a high reactivity and are electronegative.
Also Read About:

