A homogenous mixture of two or more components called a solution has particles smaller than one nanometer in size.
Solutions come in many forms, including soda water, salt and sugar solutions, and others. Every element in a solution appears as a single phase. There is particle homogeneity, or a uniform distribution of the particles. This explains why a soft drink bottle’s entire contents have the same flavor.
Properties of solution
The following are several characteristics of solutions:
- It is a uniform blend.
- With a diameter of less than 1 nm, its particles are excessively small.
- To the naked eye, the particles are invisible.
- Since particles don’t scatter light when it passes through them, the path of the light cannot be seen.
- The mixture cannot be separated from the solutes, and they do not settle. A stable solution exists.
- Filtration cannot separate the components of a mixture.
- When Kool-Aid crystal particles disperse throughout the water, the solution is produced.
- The energy of the solvent and the size of the solute particle both affect how quickly this diffusion occurs. The rate of diffusion will rise with a higher solvent temperature.
Types Of Solutions
Solvent and solute are the two elements that make up a solution.
- Solvent: The solvent is the element that dissolves the other element.
- Solute: It refers to the component(s) that dissolve in the solvents.
Typically, the solvent is present in a greater amount than the solute. The solute is less abundant than the solvent. The solute and solvent may both be in solid, liquid, or gaseous states of matter.
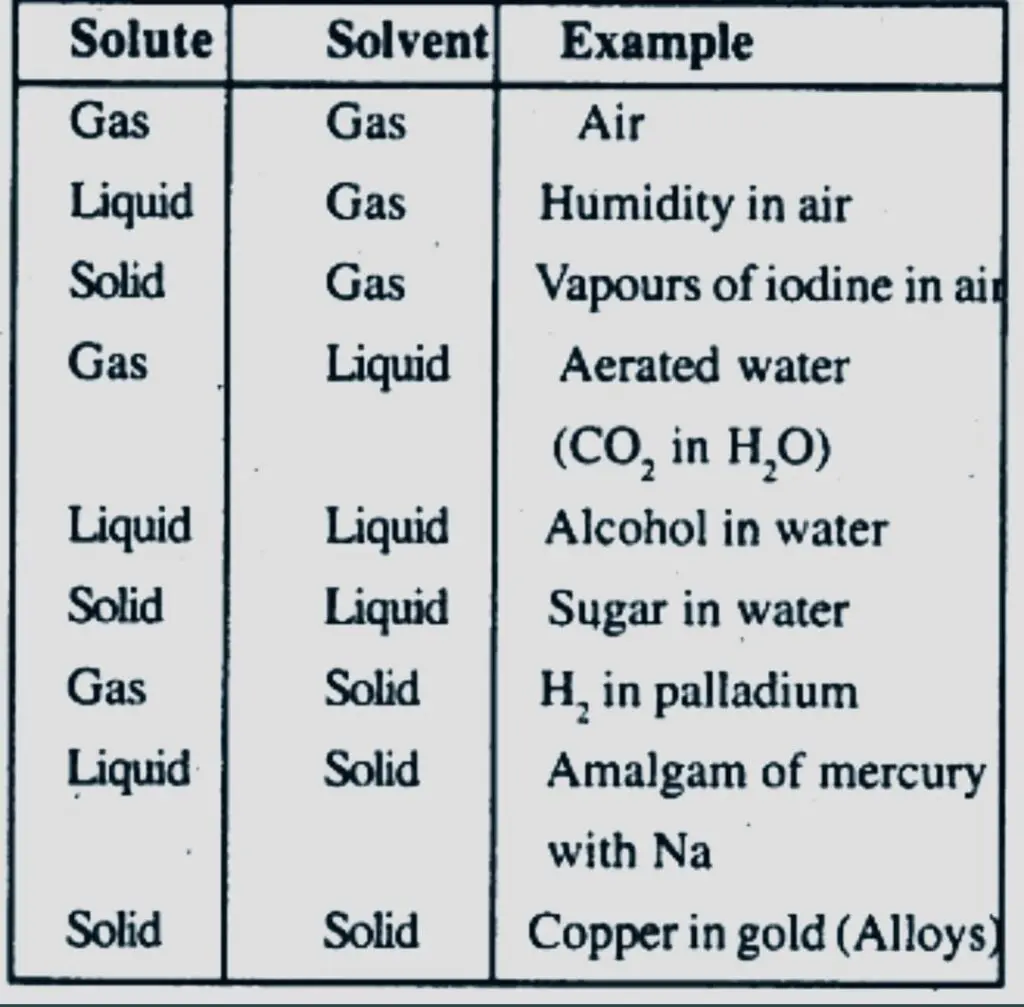
Examples of Solutions:
- The examples that follow show how a solution might contain both a solvent and a solute. A homogenous mixture of gases makes up air. Here, the solute and the solvent are both gases.
- A solution made of sugar and water is called sugar syrup. The solvent in this instance is water, while the solute is sugar.
- Iodine and alcohol are combined to make an iodine tincture. Alcohol is the solvent, whereas iodine is the solute.
Also Read About:

