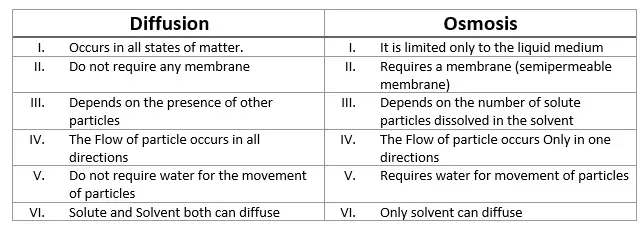
Key Differences Between Osmosis and Diffusion
There is a remarkable difference between Osmosis and Diffusion. For instance:
State of Matter and Membrane Requirement
- Diffusion occurs in all states of matter: Solid, Liquid, and Gas. Osmosis is only restricted to liquids.
- Osmosis requires a membrane known as a Semipermeable membrane. Whereas Diffusion does not require any membrane.
- In the Osmosis process, only the solvent molecules can diffuse. But in Diffusion, both solvent and solute molecules can be diffused.
Comparison can also be seen as follows in Diffusion and Osmosis: